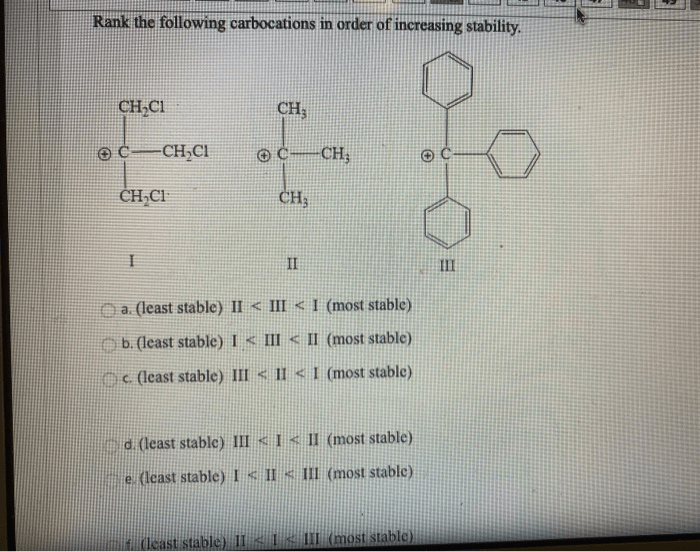
Rank the following carbocations in order of increasing stability – Carbocation stability, a fundamental concept in organic chemistry, dictates the reactivity and behavior of these crucial intermediates. In this comprehensive guide, we delve into the factors influencing carbocation stability, empowering readers with a thorough understanding of their chemical nature and reactivity.
Delving into the realm of carbocation resonance, we explore how resonance delocalizes positive charge, enhancing stability. We examine the impact of hybridization on electron density distribution and discuss the influence of inductive effects, electron-withdrawing, and electron-donating groups on carbocation stability.
Carbocation Stability Overview: Rank The Following Carbocations In Order Of Increasing Stability
Carbocation stability refers to the relative ease with which a carbocation can be formed and its resistance to further reactions. Carbocations are positively charged carbon ions that are highly reactive and can undergo various reactions, including rearrangements, additions, and eliminations.
The stability of carbocations is influenced by several factors, including:
- Resonance
- Hybridization
- Inductive effects
- Carbocation structure
Carbocation Resonance
Resonance is a phenomenon that occurs when multiple Lewis structures can be drawn for a molecule or ion. In the case of carbocations, resonance delocalizes the positive charge over multiple atoms, making the carbocation more stable. The more resonance structures that can be drawn, the more stable the carbocation.
For example, the allyl carbocation is more stable than the primary carbocation because the positive charge can be delocalized over three carbon atoms.
Carbocation Hybridization
The hybridization of the carbon atom bearing the positive charge also affects carbocation stability. Carbocations with sp 2hybridization are more stable than those with sp 3hybridization because the sp 2-hybridized carbon atom has a lower energy and is more stable.
For example, the vinyl carbocation is more stable than the ethyl carbocation because the vinyl carbocation has an sp 2-hybridized carbon atom, while the ethyl carbocation has an sp 3-hybridized carbon atom.
Inductive Effects, Rank the following carbocations in order of increasing stability
Inductive effects are the electron-withdrawing or electron-donating effects of substituents on the carbocation. Electron-withdrawing groups destabilize carbocations by withdrawing electron density from the positive carbon atom, while electron-donating groups stabilize carbocations by donating electron density to the positive carbon atom.
For example, the triphenylmethyl carbocation is more stable than the methyl carbocation because the phenyl groups are electron-donating groups.
Carbocation Structure
The structure of a carbocation can also affect its stability. Carbocations with ring strain or steric hindrance are less stable than those without these structural features.
For example, the cyclopropyl carbocation is less stable than the propyl carbocation because of the ring strain in the cyclopropyl ring.
Common Queries
What is the significance of carbocation stability in organic chemistry?
Carbocation stability plays a pivotal role in determining the reactivity and behavior of carbocations, influencing the rates and pathways of organic reactions.
How does resonance contribute to carbocation stability?
Resonance delocalizes the positive charge over multiple atoms, reducing the electron deficiency at any one carbon and enhancing stability.
What is the relationship between hybridization and carbocation stability?
Hybridization affects the distribution of electron density around the carbocation center, with sp2-hybridized carbocations being more stable than sp3-hybridized ones due to better overlap of p-orbitals.