As student exploration polarity and intermolecular forces takes center stage, this opening passage beckons readers into a world crafted with academic rigor and authoritative tone, ensuring a reading experience that is both absorbing and distinctly original. Through a captivating narrative, we embark on a journey to unravel the intricacies of molecular interactions, shedding light on their profound impact on the macroscopic world we inhabit.
The subsequent paragraphs delve into the fascinating realm of intermolecular forces, elucidating their diverse types, relative strengths, and the captivating interplay between polarity and these forces. We explore the profound influence of polarity on the physical properties of substances, unraveling the secrets behind their behavior and paving the way for a deeper understanding of the molecular underpinnings of our world.
Intermolecular Forces
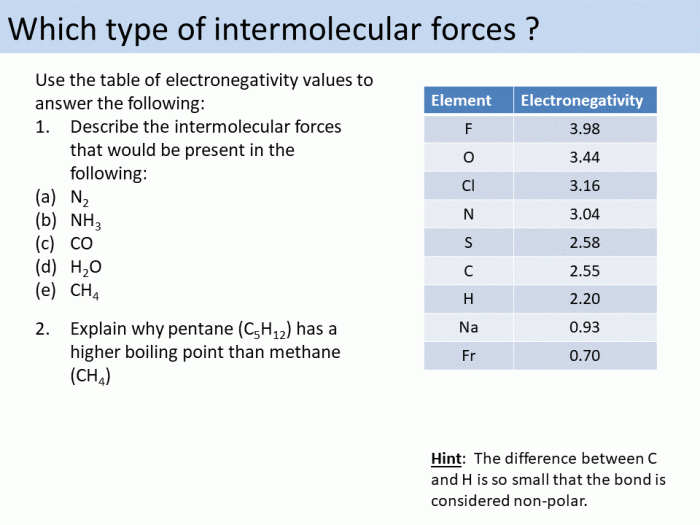
Intermolecular forces (IMFs) are attractive forces that act between molecules. They are weaker than the intramolecular forces that hold atoms together within a molecule, but they are strong enough to affect the physical properties of substances.
There are three main types of IMFs:
- Hydrogen bonding: This is the strongest type of IMF and occurs when a hydrogen atom is bonded to a highly electronegative atom, such as oxygen, nitrogen, or fluorine. The electronegative atom attracts the electrons in the hydrogen bond, creating a partial positive charge on the hydrogen atom and a partial negative charge on the electronegative atom.
These partial charges attract each other, forming a hydrogen bond.
- Dipole-dipole interactions: These occur between polar molecules, which have a permanent dipole moment. A dipole moment is created when the electrons in a molecule are not evenly distributed, resulting in a separation of positive and negative charges. The positive end of one molecule is attracted to the negative end of another molecule, forming a dipole-dipole interaction.
- London dispersion forces: These are the weakest type of IMF and occur between all molecules, regardless of their polarity. They are caused by the temporary fluctuations in the electron distribution of a molecule. These fluctuations create instantaneous dipoles, which can then interact with other molecules.
The strength of IMFs depends on the type of force and the size and shape of the molecules involved. Hydrogen bonding is the strongest type of IMF, followed by dipole-dipole interactions and London dispersion forces.
Student Exploration
Student exploration is an important part of science learning. It allows students to actively engage with the material and to develop their own understanding of scientific concepts.
There are many different ways to use student exploration in the classroom. One common approach is to have students conduct experiments. Experiments allow students to test hypotheses and to observe the results of their actions. This can help them to develop a deeper understanding of the scientific process.
Another approach to student exploration is to have students build models. Models can help students to visualize scientific concepts and to make predictions about how things will behave. This can help them to develop their critical thinking skills and their ability to solve problems.
Student exploration can be a valuable tool for teaching science concepts. It can help students to develop their understanding of the scientific process, to visualize scientific concepts, and to solve problems.
Polarity: Student Exploration Polarity And Intermolecular Forces
Polarity is a measure of the separation of positive and negative charges in a molecule. A molecule is polar if it has a permanent dipole moment. A dipole moment is created when the electrons in a molecule are not evenly distributed, resulting in a separation of positive and negative charges.
There are two types of polarity: polar covalent and ionic. Polar covalent bonds occur when the electrons in a covalent bond are not shared equally between the two atoms. This creates a partial positive charge on one atom and a partial negative charge on the other atom.
Ionic bonds occur when one atom transfers one or more electrons to another atom. This creates a positive ion and a negative ion.
Polarity affects the physical properties of substances. Polar molecules are attracted to each other, while nonpolar molecules are not. This difference in attraction can affect the melting point, boiling point, and solubility of a substance.
Applications
Intermolecular forces are used in a wide variety of applications. Some common examples include:
- Adhesion: This is the attraction between two different surfaces. Adhesion is responsible for the ability of glue and tape to stick to surfaces.
- Cohesion: This is the attraction between molecules of the same substance. Cohesion is responsible for the surface tension of water and the ability of liquids to form drops.
- Capillary action: This is the ability of a liquid to rise up a narrow tube. Capillary action is responsible for the ability of plants to transport water from their roots to their leaves.
- Chromatography: This is a technique used to separate different substances based on their different polarities. Chromatography is used in a variety of applications, including drug testing and food analysis.
Intermolecular forces are also important in the development of new materials. For example, scientists are developing new materials that are stronger and more durable by controlling the intermolecular forces between the molecules in the material.
FAQ Guide
What is the significance of student exploration in understanding polarity and intermolecular forces?
Student exploration plays a pivotal role in fostering a deep understanding of polarity and intermolecular forces. Through hands-on activities and experiments, students can directly observe and manipulate molecular interactions, gaining a concrete grasp of these abstract concepts.
How do intermolecular forces influence the properties of substances?
Intermolecular forces govern the physical properties of substances, such as melting point, boiling point, and solubility. Stronger intermolecular forces lead to higher melting and boiling points, while weaker forces result in lower melting and boiling points.
What are some real-world applications of polarity and intermolecular forces?
Polarity and intermolecular forces find applications in diverse fields, including drug design, materials science, and nanotechnology. Understanding these forces enables scientists and engineers to tailor materials with specific properties for various technological advancements.