Decay series of uranium 238 worksheet answers – Introducing the Decay Series of Uranium-238 Worksheet Answers, a definitive guide to understanding the intricacies of radioactive decay. This comprehensive resource provides a clear and engaging exploration of the decay chain, characteristics, and applications of Uranium-238, empowering learners with a deep understanding of this fundamental concept.
Delve into the fascinating world of nuclear physics as we unravel the mysteries of radioactive decay, one step at a time. With detailed explanations and practical examples, this worksheet unravels the complexities of the Uranium-238 decay series, providing a solid foundation for further exploration.
Understanding the Decay Series of Uranium-238: Decay Series Of Uranium 238 Worksheet Answers
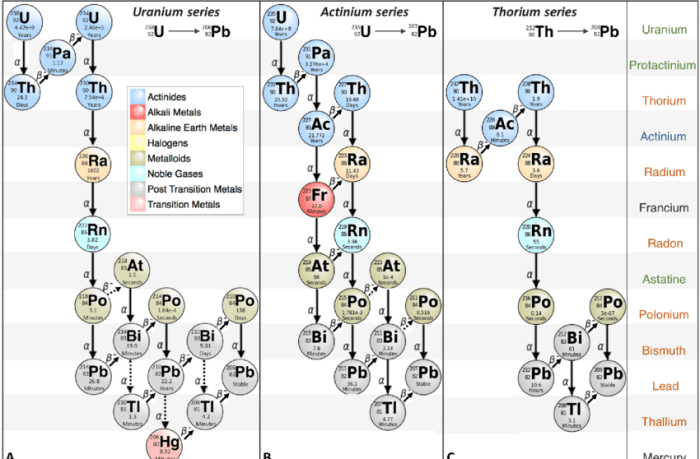
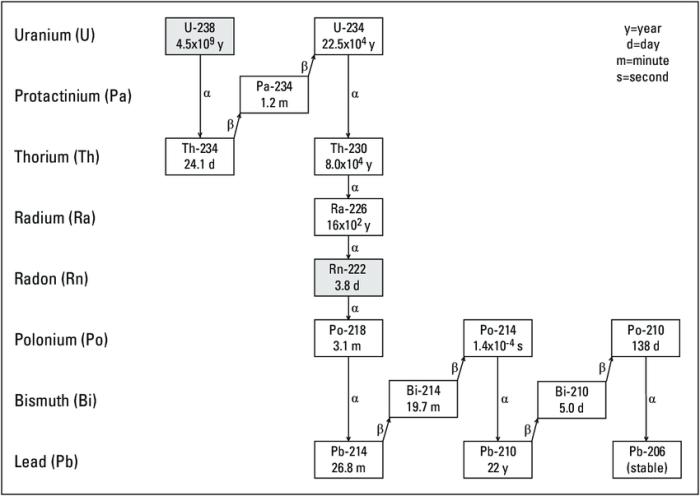
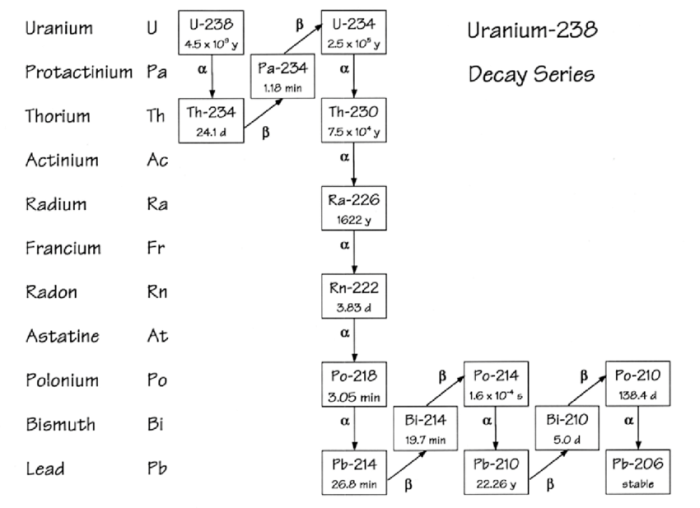
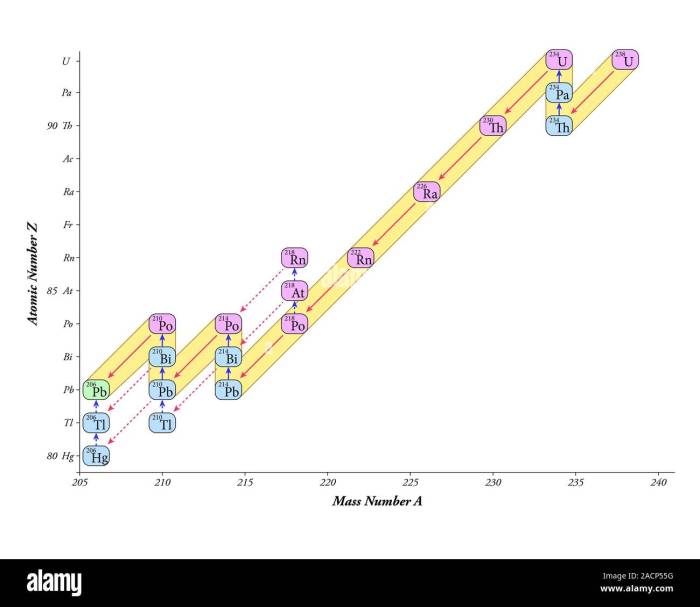
A decay series is a sequence of radioactive decays in which one unstable isotope transforms into another until a stable isotope is reached. Uranium-238 (U-238) is the heaviest naturally occurring element and undergoes a decay series involving 14 intermediate isotopes before reaching the stable isotope lead-206 (Pb-206).
The decay chain of U-238 can be represented as follows:
238U → 234Th → 234mPa → 234U → 230Th → 226Ra → 222Rn → 218Po → 214Pb → 214Bi → 214Po → 210Pb → 210Bi → 210Po → 206Pb
Each isotope in the decay series has unique characteristics and properties.
For example, U-238 has a half-life of 4.47 billion years, while Pb-206 is stable.
Frequently Asked Questions
What is the significance of half-life in radioactive decay?
Half-life is a crucial concept in radioactive decay, representing the time it takes for half of a radioactive substance to decay. It plays a vital role in understanding the decay rate and predicting the remaining activity of a radioactive sample over time.
How is the decay series of Uranium-238 used in geological dating?
The decay series of Uranium-238 is widely used in geological dating, particularly for determining the age of rocks and fossils. By measuring the ratios of different isotopes in the decay chain, scientists can estimate the time elapsed since the rock or fossil formed.
What are the practical applications of understanding the decay series of Uranium-238?
Understanding the decay series of Uranium-238 has numerous practical applications, including radioactive waste management, nuclear energy production, and medical imaging. It also provides insights into Earth’s history and the evolution of the universe.