Identify which properties could correspond to solids plasmas or both – This exploration delves into the intriguing realm of matter, examining the properties that define solids and plasmas. By identifying shared characteristics, we uncover the potential for novel materials with unique applications.
Solids, with their well-defined shape and volume, exhibit a molecular structure that governs their behavior. Plasmas, on the other hand, are characterized by high temperatures and ionization, leading to a distinct set of properties.
Properties of Solids
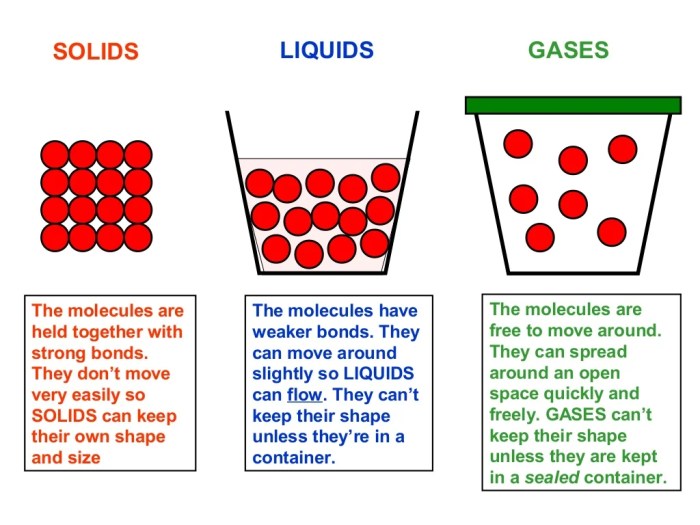
Solids are characterized by their definite shape and volume. This is because the molecules in a solid are held together by strong forces of attraction. The molecules in a solid are arranged in a regular, repeating pattern called a crystal lattice.
The crystal lattice gives solids their strength and rigidity.
Solids can be classified into two main types: crystalline and amorphous. Crystalline solids have a regular, repeating crystal lattice. Amorphous solids do not have a regular crystal lattice. Examples of crystalline solids include metals, salts, and minerals. Examples of amorphous solids include glass and plastics.
Applications of Solids, Identify which properties could correspond to solids plasmas or both
- Solids are used in a wide variety of applications, including:
- Construction
- Transportation
- Manufacturing
- Electronics
- Medicine
Properties of Plasmas
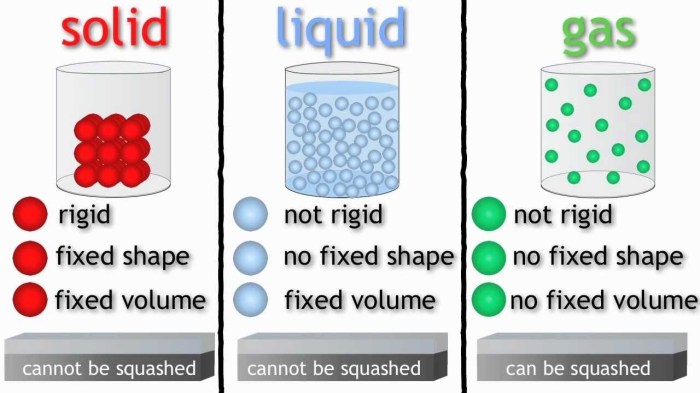
Plasmas are characterized by their high temperature and ionization. Plasmas are created when a gas is heated to a very high temperature. This causes the electrons in the gas to be stripped away from the atoms, creating a soup of free electrons and ions.
Plasmas are often called the fourth state of matter, after solids, liquids, and gases.
Plasmas are very good conductors of electricity. This makes them useful in a variety of applications, including:
- Lighting
- Plasma displays
- Plasma cutters
- Plasma engines
Properties of Solids and Plasmas: Identify Which Properties Could Correspond To Solids Plasmas Or Both
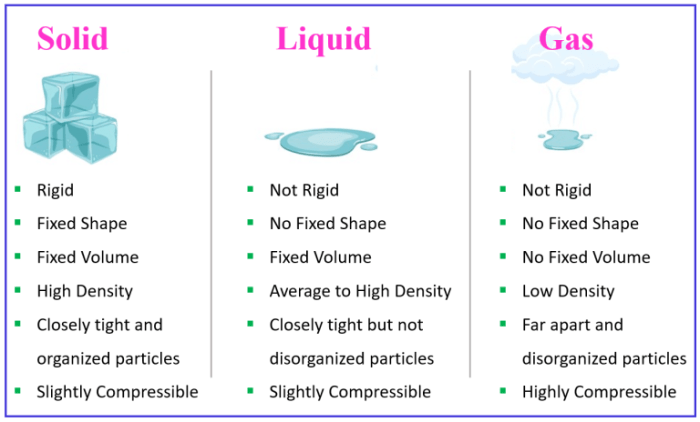
Solids and plasmas have some properties in common. Both solids and plasmas are composed of atoms or molecules. Both solids and plasmas can conduct electricity. However, there are also some key differences between solids and plasmas.
One of the key differences between solids and plasmas is their density. Solids are much denser than plasmas. This is because the atoms or molecules in a solid are packed together more tightly than the atoms or molecules in a plasma.
Another key difference between solids and plasmas is their temperature. Solids are typically much cooler than plasmas. This is because the atoms or molecules in a solid are vibrating less than the atoms or molecules in a plasma.
Case Studies

There are a number of materials that exhibit properties of both solids and plasmas. One example is a material called a semiconductor. Semiconductors are used in a variety of electronic devices, such as transistors and solar cells.
Another example of a material that exhibits properties of both solids and plasmas is a material called a superconductor. Superconductors are materials that conduct electricity without any resistance. Superconductors are used in a variety of applications, such as medical imaging and particle accelerators.
Expert Answers
What distinguishes solids from plasmas?
Solids possess a definite shape and volume due to their rigid molecular structure, while plasmas are characterized by high temperature and ionization, resulting in a fluid-like behavior.
Can materials exhibit properties of both solids and plasmas?
Yes, certain materials, known as solid-plasma hybrids, display a combination of properties from both states. These materials offer unique opportunities for applications in areas such as energy storage and sensing.