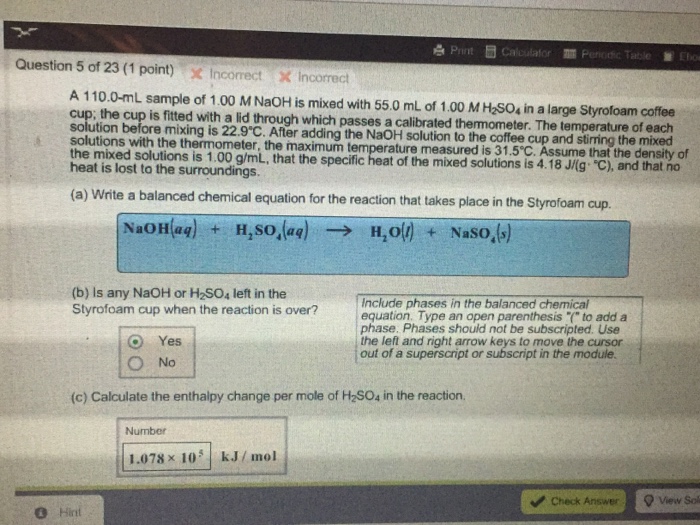
If 25.0 ml of a 0.2 M NaOH solution is at the heart of our exploration today, embarking on a journey into the realm of chemistry. NaOH, a versatile and widely used compound, presents a fascinating subject for our investigation.
Its significance in various industries, coupled with its unique chemical properties, makes this solution an intriguing topic to delve into.
NaOH, also known as sodium hydroxide or caustic soda, is a strong base that plays a crucial role in numerous chemical reactions. Its high reactivity and basicity make it an essential component in various industrial processes, such as soap and paper manufacturing.
Understanding the concentration, chemical properties, and applications of NaOH solutions is paramount for chemists, researchers, and professionals working in diverse fields.
Solution Concentration
The concentration of the NaOH solution is 0.2 M, which means that there are 0.2 moles of NaOH dissolved in every liter of solution. Molarity is a measure of the amount of solute (in this case, NaOH) present in a given volume of solution.
It is calculated by dividing the number of moles of solute by the volume of the solution in liters.
Chemical Properties of NaOH
NaOH is a strong base, which means that it readily donates hydroxide ions (OH-) in water. This makes NaOH a very reactive substance, and it can be used to neutralize acids and dissolve metals. NaOH is also used in the manufacture of soaps, detergents, and paper.
Titration Applications
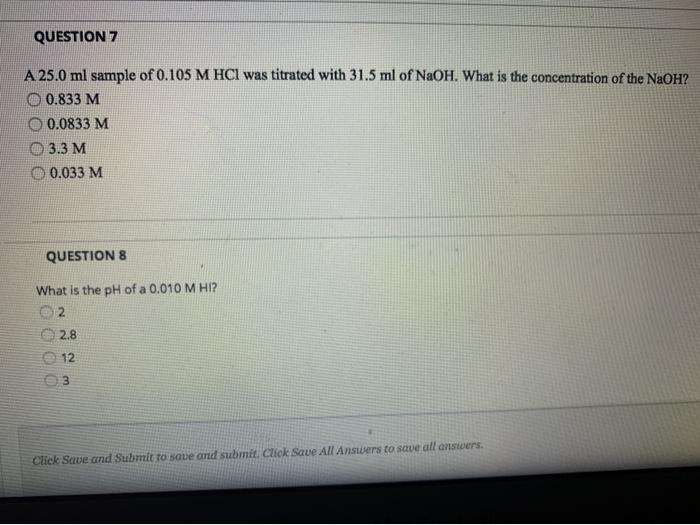
NaOH solutions are often used in titration experiments to determine the concentration of unknown acids. In a titration, a known volume of NaOH solution is added to a solution of the unknown acid until the acid is neutralized. The volume of NaOH solution required to neutralize the acid can then be used to calculate the concentration of the acid.
Safety Considerations: If 25.0 Ml Of A 0.2 M Naoh Solution
NaOH solutions can be corrosive and can cause burns if they come into contact with skin or eyes. It is important to wear gloves and eye protection when handling NaOH solutions, and to avoid contact with skin or eyes. NaOH solutions should also be stored in a cool, dry place.
Solution Preparation
To prepare a 0.2 M NaOH solution, dissolve 8.0 grams of NaOH in 1 liter of water. The amount of NaOH needed can be calculated using the following formula: “` mass of NaOH (g) = molarity (M) x volume of solution (L) x molecular weight of NaOH (g/mol) “`
pH and Neutralization Reactions
The pH of a NaOH solution is a measure of its acidity or basicity. The pH of a 0.2 M NaOH solution is approximately 13, which indicates that it is a strongly basic solution. NaOH solutions can be used to neutralize acids, and the reaction between NaOH and an acid produces water and a salt.
Spectroscopic Analysis
Spectroscopic techniques can be used to analyze NaOH solutions. UV-Vis spectroscopy can be used to measure the absorbance of NaOH solutions at different wavelengths, and this information can be used to determine the concentration of NaOH in the solution. IR spectroscopy can be used to identify the different functional groups present in NaOH solutions.
Environmental Impact
The production and disposal of NaOH can have a negative impact on the environment. NaOH is a corrosive substance, and it can damage water supplies and soil. NaOH can also react with other chemicals to produce harmful pollutants.
FAQ Summary
What is the molarity of a solution that contains 0.1 moles of NaOH in 500 ml of solution?
Molarity = 0.1 moles / 0.5 liters = 0.2 M
What is the pH of a 0.2 M NaOH solution?
pH = -log[OH-] = -log(0.2) = 13
What is the role of NaOH in titration experiments?
NaOH is used as a titrant to determine the concentration of unknown acids. It reacts with the acid in a known stoichiometric ratio, and the volume of NaOH required to reach the equivalence point is used to calculate the concentration of the acid.